
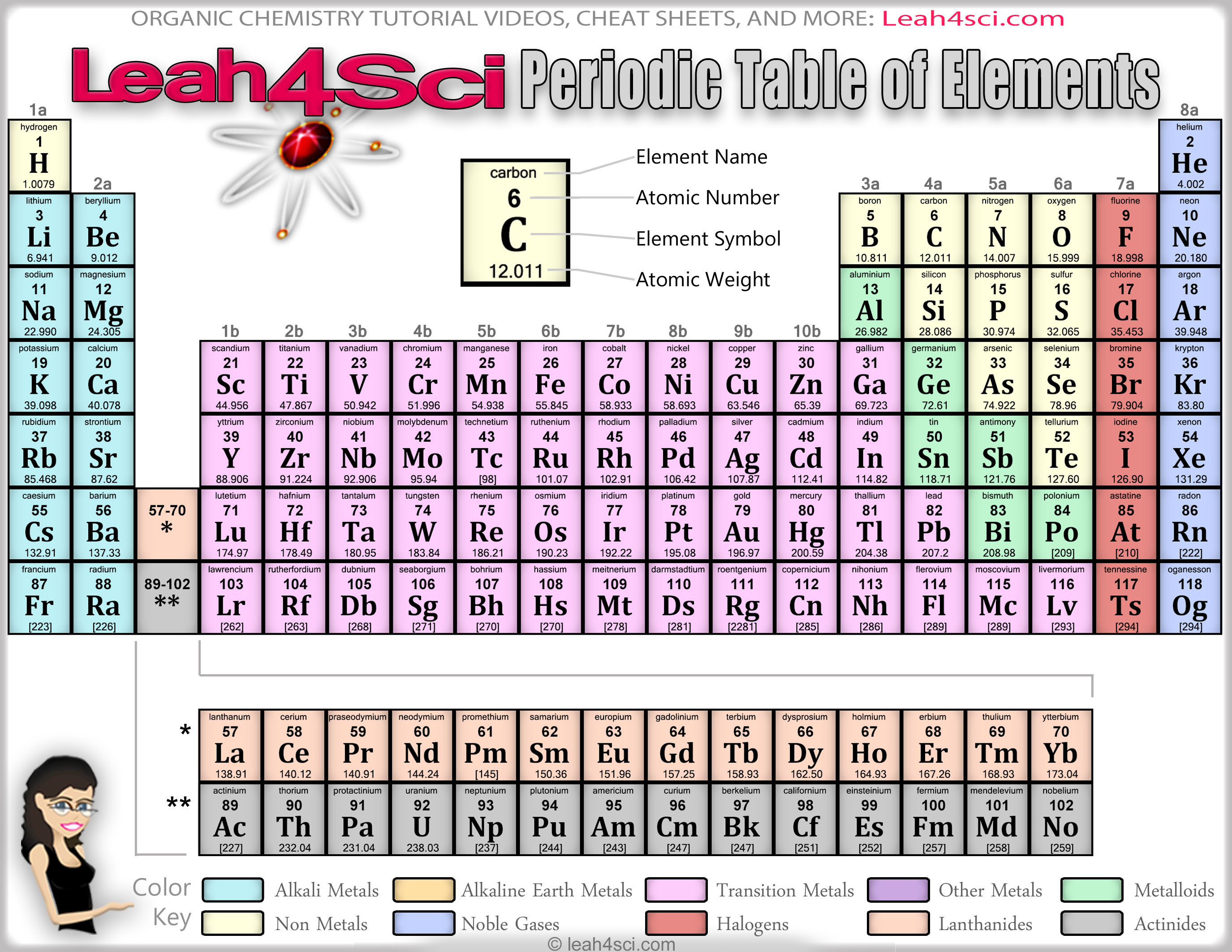


An interactive Periodic table can be found Periodic Table of the Elements, LibreTexts. The semimetals lie along a diagonal line separating the metals and nonmetals. The metals are on the bottom left in the periodic table, and the nonmetals are at the top right. Classification of the first twenty elements in the periodic table on the basis of the number of outer electrons. 1: The Periodic Table Showing the Elements in Order of Increasing Z.The periodic table as a list of elements arranged so as to demonstrate trends in their physical and chemical properties.Comparison of Mendeleev’s table with the modern periodic table.Describe and model the structure of the atom in terms of the nucleus, protons, neutrons and electrons comparing mass and charge of protons neutrond and electrons. 1.6.8 recall that elements with similar properties appear in the same group (for example Group 1 (I) and Group 2 (II) are groups of reactive metals, Group 7 (VII) is a group of reactive non-metals and Group 0 is a group of non-reactive non-metals)….Unit C1: Structures, Trends, Chemical Reactions, Quantitative Chemistry and Analysis.1.6.8 recall that elements with similar properties appear in the same group (for example Group 1 (I) and Group 2 (II) are groups of reactive metals, Group 7 (VII) is a group of reactive non-metals and Group 0 is a group of non-reactive non-metals),….Unit 1: Structures, Trends, Chemical Reactions, Quantitative Chemistry and Analysis.(d) general trends in ionisation energy, melting temperature and electronegativity across periods and down groups.(a) elements being arranged according to atomic number in the Periodic Table.Unit 1: THE LANGUAGE OF CHEMISTRY, STRUCTURE OF MATTER AND SIMPLE REACTIONS.(l) the similarities and trends in physical and chemical properties of elements in the same group as illustrated by Group 1 and Group 7.(i) metals being found to the left and centre of the Periodic Table and non-metals to the right, with elements having intermediate properties appearing between the metals and non-metals in each period.(h) elements being arranged in order of increasing atomic number and in groups and periods in the modern Periodic Table, with elements having similar properties appearing in the same groups.1.2 ATOMIC STRUCTURE AND THE PERIODIC TABLE.Unit 1: CHEMICAL SUBSTANCES, REACTIONS and ESSENTIAL RESOURCES.(k) the similarities and trends in physical and chemical properties of elements in the same group as illustrated by Group 1 and Group 7.(h) metals being found to the left and centre of the Periodic Table and non-metals to the right, with elements having intermediate properties appearing between the metals and non-metals in each period.(g) elements being arranged in order of increasing atomic number and in groups and periods in the modern Periodic Table, with elements having similar properties appearing in the same groups.2.2 ATOMIC STRUCTURE AND THE PERIODIC TABLE.RSC Yusuf Hamied Inspirational Science Programme.Introductory maths for higher education.The physics of restoration and conservation.


 0 kommentar(er)
0 kommentar(er)
